Download PDF version here
Plant Nutrition : Essential elements for plants, what they are and why plants need them.
By Grace Lin
AEssense Applications Scientist
Plant nutrition is the study of chemical elements and compound that are necessary for plant growth, and the study of how plants obtain and use mineral nutrients. We do know that plants get “food” by the process of photosynthesis, which, to be more specific, is the process of converting water (H2O) and carbon dioxide (CO2) into carbohydrates (or “sugar”, C6H12O6), oxygen, and water using the energy from the light. However, there are a lot more chemical elements that plants cannot live without, and plants typically absorb these elements from their roots.Plant essential elements are defined to meet one of the 3 criteria:
- Without the element, it is impossible for the plant to complete its life cycle
- The function of the element is specific and cannot be replaced by any other element
- The element is directly involved in essential metabolite or required for the function of an enzyme system.
For example, the following graph is the chemical structure of chlorophyll a and chlorophyll b, where plants conduct photosynthesis:
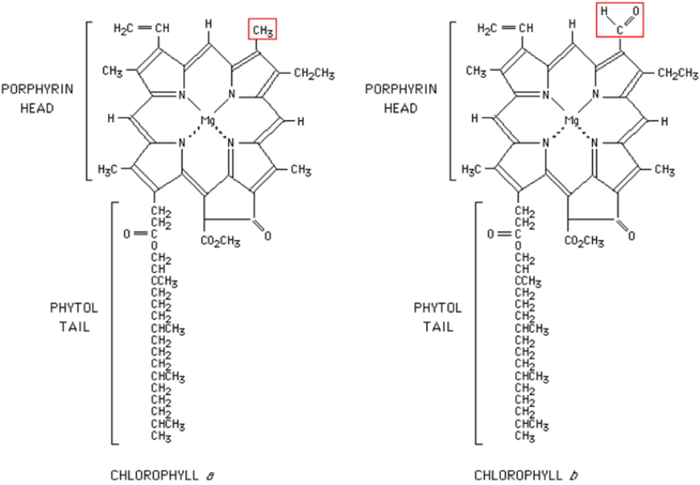
You can notice that most parts of the structure are composed by carbon (C), oxygen (O) and hydrogen (H). The red circle in the center marks the critical structure (the official name is “porphyrin-head”) of chlorophyll, composed by 4 nitrogen (N) and one magnesium (Mg). Without these two elements, plants cannot form chlorophyll and cannot conduct photosynthesis successfully.
That’s why we typically see yellowing leaves when nitrogen or magnesium are not sufficient: the plant will start to degrade the chlorophyll from the old leaves. Understanding the role of each element plays in plant physiology will also help us to diagnose nutrient deficiency in cultivation and make better decisions in adjusting a nutrient delivery program.
Plants contain about 85-90% of water. When we take a plant and dry it completely, we usually get the dry weight that is about 10-15% of the original weight. If we analyze the dry weight, about 90% of it consists of 3 elements: carbon (C), oxygen (O) and hydrogen (H).
The remaining elements can be separated into two groups depending on their dry weight: macronutrient (0.2%-4% in dry weight), which plants need in a large quantities, and micronutrient (<0.02% in dry weight), which plants need in a small quantities.
About 18 chemical elements are considered essential nutrients for plants, including 9 macronutrients: carbon (C), oxygen (O), hydrogen (H), nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg) and sulfur (S), and 9 micronutrients: iron (Fe), chlorine (Cl), manganese (Mn), boron (B), sodium (Na), zinc (Zn), copper (Cu), molybdenum (Mo) and nickel (Ni). Silicon (Si) is also considered essential nutrients for some plant species.
Functions of essential nutrients for plants
The essential nutrients can be classified into 4 groups according to their physiological function:
- Nutrients that are part of carbon compounds: Nitrogen (N) and Sulfur (S). Plants assimilate these two nutrients through biochemical reactions to create some of the most important compound in the plants such as nucleic acids (the component of DNA) and proteins.
- Nitrogen (N): essential element to form amino acids, proteins, nucleic acids, coenzymes and chlorophyll.
- Sulfur (S): component of several amino acids, proteins, coenzyme A and some vitamins.
- Nutrients that are important in energy storage reactions or in maintaining structural integrity: Phosphorus (P), Silicon (Si) and Boron (B).
- Phosphorus (P): component of important compounds of plant cells, including the energy compounds (such as ATP) for respiration and photosynthesis, and the phospholipids that make up the plant membranes.
- Silicon (Si): contribute to cell wall rigidity and elasticity.
- Boron (B): Involved in cell wall structure, cell elongation and nucleic acid metabolism.
- Nutrients that remain in ionic form. They may be found in the solutions inside the cells (cytosol) or bound in carbon-containing compounds.
- Potassium (K): potassium presents in plants as the cation K+, and plays an important role in regulation of the osmotic potential of plant cells. It is also required to active for more than 40 enzymes.
- Calcium (Ca): calcium ions (Ca+) participated in the synthesis of cell walls and the process of cell division. Calcium is required to maintain cell membrane integrity and acts as a second messenger in metabolic regulation.
- Magnesium (Mg): an essential component of chlorophyll molecule. Required by many enzymes involved in respiration, photosynthesis and the synthesis of DNA.
- Chlorine (Cl): found in plants as chloride ion (Cl-). It is required for the part of the photosynthesis reaction, when water is split and oxygen is produced.
- Manganese (Mn): manganese ions (Mn2+) activate many enzymes in plant cells involved in fatty acid synthesis and DNA formation. It is also part of the photosynthesis reaction when water is split and oxygen is produced.
- Sodium (Na): particularly important for C4 and CAM plants (two other different photosynthesis pathways, for most plant species, they conduct C3 photosynthesis) as it is involved in carbon fixation pathway. Substitutes for potassium in some functions.
- Nutrients that are involved in “redox reactions”, which means these elements can undergo reversible oxidations and reductions (for example, Fe2+Fe3+) and play important roles in electron transfer (e+) and energy transformation in most of the important reactions.
- Iron (Fe): an essential part of cytochromes, which acts as electron carriers in photosynthesis, respiration and nitrogen fixation. Also involved in the synthesis of chlorophyll.
- Zinc (Zn): involved in the formation of plant hormone IAA. Activate many enzymes in various physiology reactions.
- Copper (Cu): like iron, copper is involved in redox reactions from Cu+ to Cu2+, which transfers electron during the light reactions of photosynthesis.
- Nickel (Ni): essential for nitrogen fixation as it involves in the conversion of nitrate to ammonium. Urease is the only known nickel-containing (Ni2+) enzyme in higher plants.
- Molybdenum (Mo): an essential component of several enzymes and acts as electron carriers in nitrogen fixation (Mo4+ through Mo6+).
When growing with artificial substrates or in hydroponic systems, it is critical that ALL the micronutrients need to also be available in the water. Soil fertilizers and hydroponic plant nutrients are basically the same things in different combinations.
In general, when the nutrients related to chlorophyll and photosynthesis are not sufficient, we see a various degrees of tissue yellowing (chlorosis) with different patterns. For more details about the symptoms of each nutrient elements, please see “nutrient deficiencies”.
References:
Taiz and Zeiger, 2010, Plant Physiology, Sinauer Associates, 5th Edition.
Howard M. Resh, 2012, Hydroponic Food Production, CRC Press, 7th edition.